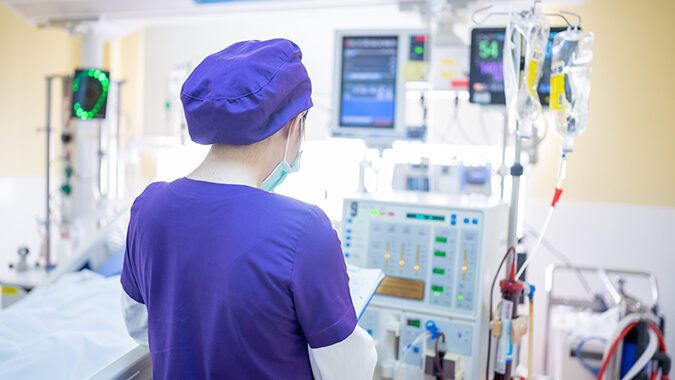
The U.S. Food and Drug Administration (FDA) has granted fast track designation for Merck’s investigational anticoagulant therapy, MK-2060, for reducing the risk of major thrombotic cardiovascular events in patients with end-stage renal disease.
MK-2060 is a monoclonal antibody now being evaluated in a Phase 2 study in patients with end-stage renal disease (ESRD) who are on hemodialysis, Merck said Tuesday in a statement announcing the FDA designation. Fast track is a process designed to facilitate the development and expedite the review of drugs that treat serious conditions and fill an unmet medical need.
Dr. Eliav Barr, senior vice president and head of global clinical development at Merck Research Laboratories, sad MK-2060 has the potential for preventing blood clots in patients with advanced kidney disease.
“At Merck we are focusing our efforts where the needs are greatest, and we believe we have a significant opportunity with MK-2060 for the potential prevention of thrombosis in patients with advanced forms of kidney disease,” Dr. Barr said.
“We are encouraged by this fast-track designation because additional anticoagulation medicines are urgently needed for patients with ESRD who are susceptible to high rates of life-threatening thrombotic events as well as high bleeding risk,” Dr. Barr said. “Today there is no anticoagulation standard of care for such patients.”
MK-2060, administered intravenously, is designed to work through a dual mechanism of action both blocking the activation of Factor XI as well as the downstream activity of activated protein. MK-2060 is being investigated in a Phase 2 study to evaluate the efficacy and safety of two different doses of MK-2060 in participants with ESRD receiving hemodialysis via an arteriovenous graft. Data from this study will be used to aid dose selection in future studies.